Some cool Weight loss images:
Image from page 175 of “Memoirs and proceedings of the Manchester Literary & Philosophical Society” (1888)
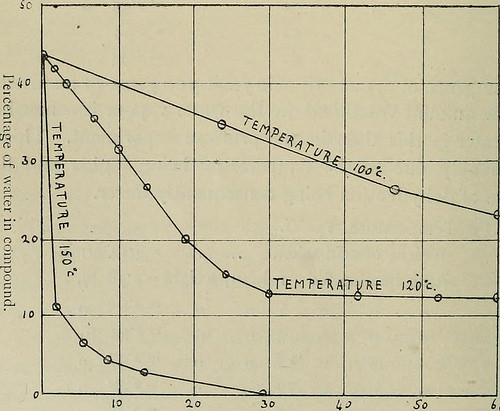
Image by Internet Archive Book Images
Identifier: memoirsproceedin55manc
Title: Memoirs and proceedings of the Manchester Literary & Philosophical Society
Year: 1888 (1880s)
Authors: Manchester Literary and Philosophical Society
Subjects: Manchester Literary and Philosophical Society
Publisher: Manchester : The Society
Contributing Library: American Museum of Natural History Library
Digitizing Sponsor: Biodiversity Heritage Library
View Book Page: Book Viewer
About This Book: Catalog Entry
View All Images: All Images From Book
Click here to view book online to see this illustration in context in a browseable online version of this book.
Text Appearing Before Image:
) 1) !) 118 ,, ,, =274 ,, » ;, â¢, 125 ,, ,, =274 ,, 3*89 grms. of orthoboric acid yield 276 grms. of meta- boric acid. The loss of acid through volatilisation is very small. (2) Temperature, i2o°C. Weight of acid taken =422graiP. After heating for i hour, weight =4Tf â 1 J h ours 11 = 4-04 6 11 ) = 3-S7 10 11 M = 371 14 11 )) = 3 49 19 >) )) = 323 25 r 11 = 3-01 30 11 11 = 2-95 42 )) )> -2-94 52 )) )) = 2-94 422 grms. of orthoboric acid yield 3 00 grms. of meta acid.The amount volatilised in the water vapour is somewhatgreater in this than in the previous experiment. This isprobably due to the temperature being higher and therate of dehydration being consequently faster. (3) Temperature, 150C. Weight of acid taken … … =4i4grms. After heating for i hour, weight =278 ,, J> )i )J 5 2 DO ,, >> )) )) >> )) ~ 2 54 )) )i )) )) 3 =2 47 11 )) )) )) 29 )) 1) 232,, 5j J) jj 37 )) !> ~ 2 20 â Mult, The Boi ic Acids. So ko – ie c 1 3 Time in Day-Iv.^. I.
Text Appearing After Image:
1o io Time in Hours. Mi^ 2. MancJicstii- Mcinoirs, Vol. h. (1911), No, 10- 5 4 14 grms. of orthoboric acid yield 292 grms. ofmetei acid and 264 grms. of p)-ro acid. In this case theloss through volatiHsatiou is much greater, the finalproduct weighing less than the tlieoretical amount forboric anh}dride. It would, therefore, seem that theamount volatilised depends upon the rate of heating.These three experiments, only calculated to show thepercentage of water present in the compound from timeto time, are shown graphically in Figs, i and 2, fromwhich it will be seen that only one break exists in thedeh)-dration curve. It will be noticed in Figs, i and 2that the break in the curve occurs with a lower percentageof water as the temperature at which the experiment wasconducted was raised. This is due to volatilisation of theortho acid in the water vapour. The difference between thepercentage of water present in the compoutid when thebreak takes place and the theoretical value for metaboric
Note About Images
Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability – coloration and appearance of these illustrations may not perfectly resemble the original work.
Steven F. Udvar-Hazy Center: map of the museum

Image by Chris Devers
This is the on-site version of the facility map, also available (and probably more useful to you, reader) as this PDF document: Steven F. Udvar-Hazy Center: Boeing Aviation Hangar & James S McDonnell Space Hangar.